|
|
|
New Type of Flu Vaccineby Vincent RacanielloWednesday, August 19, 2009 at 11:01 AM EDT
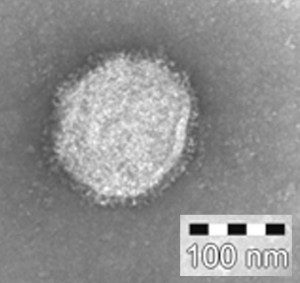
If you have been taking influenza 101, you know that new virus particles are produced in infected cells by budding. During this process, the membrane bulges from the cell and is eventually pinched off to form a free particle. These virus particles contain the viral RNA segments, and an assortment of viral proteins including PA, PB1, PB2, NP, M1, M2, HA, and NA. But not all of those viral proteins are needed to produce an influenza virus particle. When only the viral HA, NA, and M1 proteins are synthesized in cells, particles are released from cells that look very much like influenza virions (illustrated). These are called ‘virus-like particles’ because they resemble influenza viruses, but lack the viral genome and many viral proteins. Influenza virus-like particles are not infectious, but they are immunogenic: when injected into animals, they induce the production of anti-viral antibodies that can block infection. In one study, virus-like particles were produced in cultured insect cells by using an insect virus vector – a baculovirus – to deliver genes encoding the influenza HA, NA, and M1 proteins. Mice inoculated with these virus-like particles were protected after challenge with infectious virus. More recently, mice vaccinated with virus-like particles produced with proteins from an H5N1 avian strain were protected against challenge with lethal H5N1 viruses. These findings suggest that virus-like particles could be used in humans to protect against influenza infection. They offer a number of advantages over the current influenza virus vaccines, most of which are prepared by growing virus in embryonated chicken eggs. Viral infectivity is destroyed with formalin, and the virions are then disrupted with detergents. Virus-like particle vaccines would not require these treatments, and would be available to individuals with egg allergies. Some influenza virus vaccines are produced in cell culture, but these are also treated to eliminate infectivity. Another important advantage of the virus-like particle vaccine is that it can be produced relatively rapidly: within weeks, compared to months for egg-produced vaccines. This property would be especially useful when new pandemic strains emerge. For example, the swine-origin H1N1 influenza virus emerged in the spring of 2009, and vaccine manufacturers are scrambling to have a product ready for the fall. Because an influenza virus-like particle vaccine is a new type of vaccine, many years of testing in animals and in humans will be required before it can be used. Some of the questions that must be addressed include the safety of the vaccine in humans, whether the anti-viral antibody repertoire induced by the vaccine is sufficiently broad, and of course whether immunization confers efficient protection against challenge in the majority of recipients. I am particularly curious about how an influenza virus-like particle vaccine would compare with the infectious, attenuated influenza vaccine, Flumist. This intranasally-administered vaccine mimics a natural infection and has been shown to be more effective in preventing influenza than inactivated vaccine. While virus-like particle vaccines are an attractive option, they are not infectious and therefore might not induce the same antibody repertoire as would an infectious virus. A disadvantage of Flumist is that it is produced in eggs. If influenza virus-like particles prove safe and efficacious in humans, they could replace the egg-grown, inactivated vaccines. Pushko, P., Tumpey, T., Bu, F., Knell, J., Robinson, R., & Smith, G. (2005). Influenza virus-like particles comprised of the HA, NA, and M1 proteins of H9N2 influenza virus induce protective immune responses in BALB/c mice Vaccine, 23 (50), 5751-5759 DOI: 10.1016/j.vaccine.2005.07.098 Bright, R., Carter, D., Crevar, C., Toapanta, F., Steckbeck, J., Cole, K., Kumar, N., Pushko, P., Smith, G., Tumpey, T., & Ross, T. (2008). Cross-Clade Protective Immune Responses to Influenza Viruses with H5N1 HA and NA Elicited by an Influenza Virus-Like Particle PLoS ONE, 3 (1) DOI: 10.1371/journal.pone.0001501 This article originally appeared on virology blog. |
|